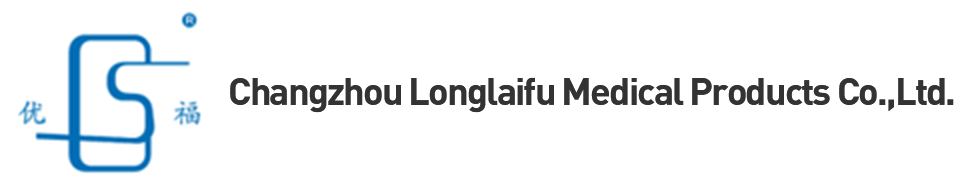How to distinguish between medical and non-medical masks
Masks can be divided into two categories: medical/non-medical. As the name suggests, medical masks are mainly used for medical protection and can be divided into three types: medical protection, medical surgery and disposable medical treatment. Non-medical masks, also known as personal protective masks, can be subdivided into anti-accumulation according to their application. Particulate matter and daily protection. Masks for different uses have different technical requirements and different application ranges
Release time:
2020-07-17
Source:
Author:
Masks can be divided into two categories: medical/non-medical. As the name suggests, medical masks are mainly used for medical protection and can be divided into three types: medical protection, medical surgery and disposable medical treatment. Non-medical masks, also known as personal protective masks, can be subdivided into anti-accumulation according to their application. Particulate matter and daily protection. Masks for different uses have different technical requirements and different application ranges
2. Use appearance and packaging information to distinguish medical/non-medical masks
Distinguish by mask structure
Solved by the filter valve. Masks with filter valves are usually not medical masks. For example, Article 4.3 of the Chinese medical protective mask standard GB 19803-2010 clearly stipulates that "masks should not have an exhalation valve", so that droplets, microorganisms, etc. can be exhaled through the exhalation valve, thereby harming others. It is permitted for civilian masks to have exhalation valves. The exhalation valve can reduce the exhalation resistance, which is advantageous for the operator to work for a long time.
Distinguish by the information on the outer packaging
The minimum packaging of mask products sold through formal channels should include information such as the product name, implementation standards and protection level. The information expressed by these merchants can be used as a point of distinction. For example, if the product name contains "Medical" (medical) or "Surgical" (surgical) and "Medical" (medical) in English, it can usually be regarded as a medical mask.
3. Applicable standards distinguish between medical/non-medical masks.
Different standards and certification requirements apply to medical masks in different countries. Businesses and individuals can be distinguished on the basis of the country where the product is imported and the applicable standards for the product. Applicable product standards and certification information can be obtained from the product packaging or manufacturer. Get a test report or certificate.
Export to the United States
Medical masks are medical devices in the United States that are governed by the "Standard Specification for Materials Performance of Medical Masks" (ASTM F2100) and are administered by the U.S. Food and Drug Administration (FDA). They must be registered through 501K or other channels recently announced by the FDA to obtain factory registration, and medical devices can only be marketed in the United States after they are marketed. Therefore, mask packages exported to the United States or test reports or certificates with the above contents can be regarded as medical masks.
Non-medical masks exported to the United States are not covered by Announcement 5 of 2020, but companies should note that products need to be registered with NIOSH in order to be marketed in the United States.
Export to EU
EU medical/non-medical masks must bear the CE mark, but the applicable standards are different.
Medical masks belong to Class I equipment in the European Union and are divided into non-sterile and sterile Class I. The trademark must be affixed on CE according to the European Union Medical Device Directive 93/42 / EEC(MDD) or the European Union Medical Device Regulation EU2017/745(MDR), the corresponding standard is EN14683, and the mask package or test report or certificate exported to the European Union.
Containing the above, can be considered a medical mask.
It should be noted that different conformity assessment methods are used in the EU depending on the sterile/non-sterile status of the mask. Non-sterile medical mask companies are only required to make a CE self-conformity declaration and do not need to be certified by a designated agency. After preparing the relevant documents and test reports, you can complete the declaration of conformity yourself. Sterile medical masks must also be CE certified by an authorized certification body.
Non-medical masks exported to the EU are not medical devices, but must meet the requirements of the EU Personal Protective Equipment Regulation EU2016/425(PPE). CE certification and certification are issued by authorized certification bodies. The corresponding standard is EN149.
Export to other countries and regions
Mask products exported to other countries and regions can be judged with reference to the Chinese standard test certificates and registration information provided by them. There are three medical mask standards in China, which are GB 19083-2010,YY 0469-2011,YY / T 0969-2013. The use of masks produced by these three standards can be judged as medical masks.
Latest information
NEWS INFORMATION